Pillar Biosciences’ OncoReveal CDx Test Receives Expanded FDA Approval
Pillar Biosciences, in collaboration with Illumina, has achieved a significant milestone with the expanded FDA approval of its OncoReveal CDx pan-cancer in vitro diagnostic test this week. Initially approved in 2021 for guiding specific therapies in non-small cell lung and colorectal cancer patients, this recent approval marks a pivotal moment in cancer diagnostics. The OncoReveal CDx test is now sanctioned as a general solid tumor profiling test, enabling hospitals and commercial labs equipped with Illumina’s MiSeq Dx next-generation sequencing platform to adopt it as part of their standard oncology testing repertoire.
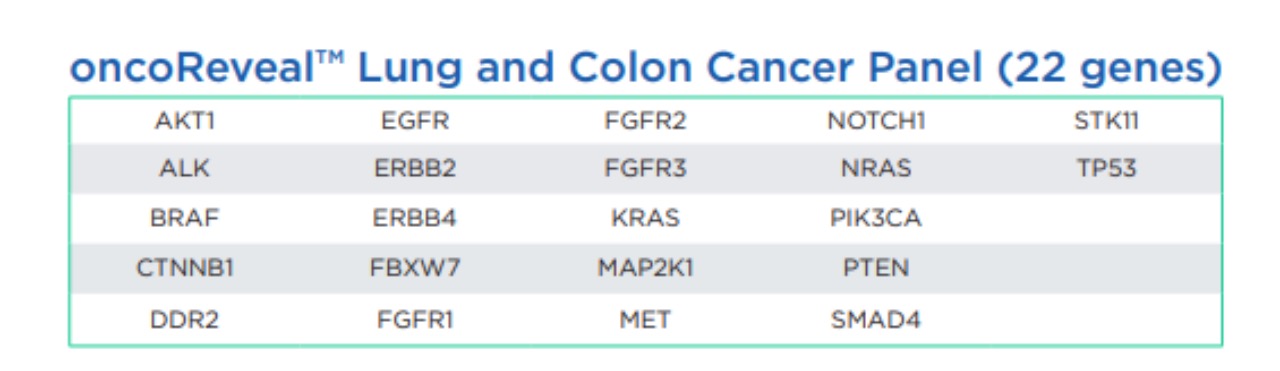
Previously limited to guiding EGFR- and KRAS-targeted therapies, the OncoReveal CDx test now offers a broader application, capable of profiling a wide range of solid tumors. This expanded utility enhances its clinical value, empowering healthcare providers with a comprehensive tool for tumor characterization and treatment decision-making.
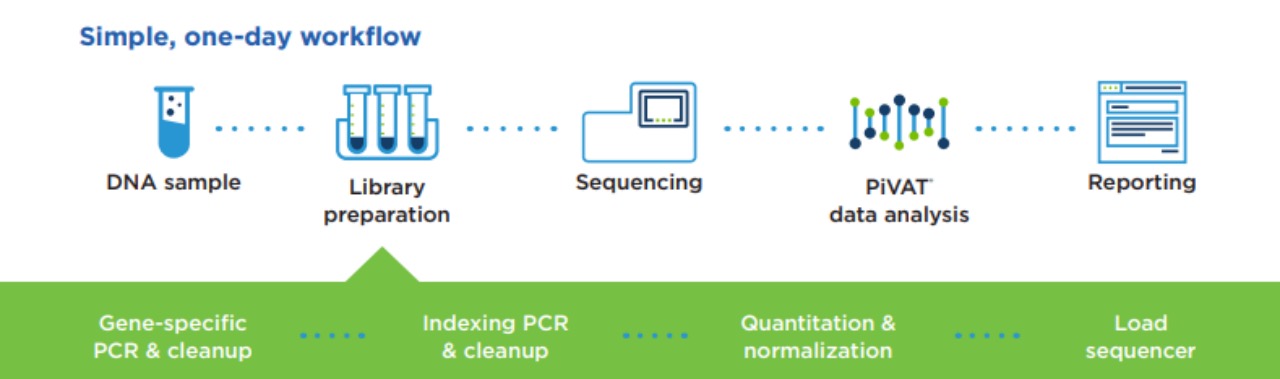
At the core of OncoReveal CDx is Pillar’s innovative amplicon-based SLIMamp target enrichment chemistry. This technology enables selective amplification of DNA regions of interest while blocking unwanted sections, ensuring accurate and reliable results. Moreover, with the ability to process 46 samples in just 48 hours, OncoReveal CDx offers a rapid turnaround time, crucial for timely patient management.
The partnership between Pillar Biosciences and Illumina extends beyond the US market, with a global distribution agreement aimed at making Pillar’s oncology assays available worldwide. This strategic collaboration underscores a shared commitment to advancing cancer diagnostics and personalized medicine on a global scale.
With FDA approval secured and a robust distribution network in place, Pillar Biosciences is poised to drive further innovation in oncology diagnostics. Continued research and development efforts promise to expand the clinical utility of OncoReveal CDx and address unmet needs in cancer care.
The expanded FDA approval of Pillar Biosciences’ OncoReveal CDx test marks a significant advancement in cancer diagnostics, offering healthcare providers a versatile tool for solid tumor profiling. With its rapid turnaround time and broad applicability, OncoReveal CDx has the potential to revolutionize treatment decision-making and improve outcomes for cancer patients worldwide.