Roche CDx for Enhertu in Breast Cancer Nabs CE Mark
Roche disclosed the receipt of the CE Mark for the VENTANA® HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx* for the determination of metastatic breast cancer patients with minimal HER2 expression, for whom ENHERTU® (trastuzumab deruxtecan) could be contemplated as a specialized therapy. The diagnostic, which goes by the name PATHWAY in the United States, was granted US Food and Drug Administration (FDA) authorization in October 2022. ENHERTU is a precisely formulated HER2-targeted antibody-drug conjugate (ADC) being collaboratively developed and marketed by Daiichi Sankyo and AstraZeneca.

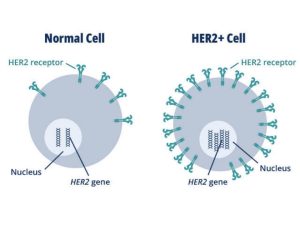
HER2 is a receptor protein that promotes rapid growth of cancer cells. To ascertain a patient’s HER2 status, pathologists assess, or rate, the amount of HER2 protein manifested in breast cancer tissue samples. If a patient’s tumor exhibits elevated levels of HER2, the patient is deemed HER2-positive and might be considered for HER2-specific therapy. Nonetheless, half of all individuals with metastatic breast cancer manifest minimal levels of HER2, which historically categorized them as HER2-negative.
“We are thrilled to prolong our innovation in breast cancer diagnostics via pivotal tests like this one, facilitating the recognition of individuals with HER2-low status,” stated Jill German, Head of Pathology Lab at Roche Diagnostics. “With this broadened authorization of our diagnostic, we’re elated that additional metastatic breast cancer patients globally might be accurately identified and possibly eligible for this specialized therapy.”
The VENTANA HER2 (4B5) diagnostic now encompasses a scoring algorithm that aids pathologists in spotting “low expressors” of HER2, granting a HER2 low status to this cohort of patients. With this diminished cutoff, the diagnostic is capable of pinpointing patients who might derive benefits from ENHERTU as a therapeutic option.
Breast cancer has eclipsed lung cancer as the most frequently diagnosed cancer, with an estimated 2.3 million new instances identified worldwide each year. More than 685,000 individuals succumb to breast cancer annually.4,5
The CE Mark of the new HER2-low indication broadens the intended application for Roche’s established, on-market VENTANA HER2 (4B5) diagnostic, providing prompt, explicit, and assured outcomes. The introduction underscores Roche’s dedication to persisting in innovating integrated, significant medical value solutions that contribute to the progression of personalized healthcare.
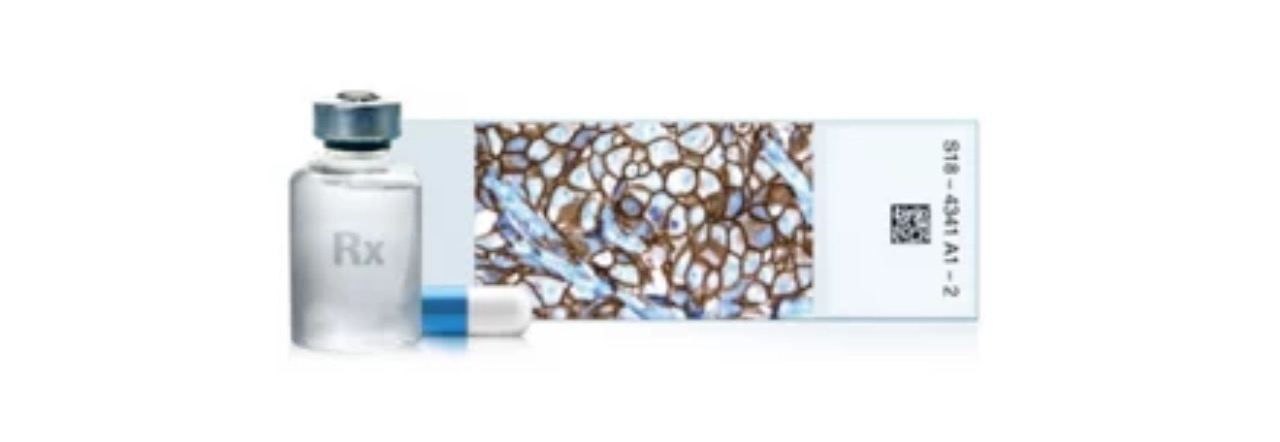
About VENTANA HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx
Roche’s ready-to-use VENTANA HER2 (4B5) Rabbit Monoclonal Primary Antibody RxDx, utilized in conjunction with the fully automated BenchMark IHC/ISH slide staining device, normalizes all immunohistochemistry (IHC) procedures from baking to staining, and diminishes the risk of human mistakes.5 It additionally reduces inherent variability stemming from individual reagent dilution and other practices observed in manual and semi-automated IHC techniques. The Roche HER2 (4B5) clone consistently achieves top proficiency assessment scores relative to other clones6 and shows high agreement with HER2 FISH7,8, enabling laboratories to utilize the most broadly adopted and dependable HER2-IHC primary antibody.