Roche wins FDA approval for first molecular malaria blood donor screening test
Malaria, a mosquito-borne disease, is caused by intraerythrocytic parasites belonging to the genus Plasmodium. Five Plasmodium species are known to cause disease in humans – P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi. Among these, P. falciparum is the most transmitted and is responsible for fatalities around the world. In 2022, malaria was transmitted in 85 countries, causing approximately 249 million cases and 608,000 deaths. While malaria transmission is rare within the U.S., ten cases of locally-transmitted malaria were reported in four states in 2023. Approximately 2000 clinical cases are reported annually in the U.S., almost all due to infections acquired outside the country. Malaria can also be transmitted by transfusion of blood, blood products, and solid organ transplantation collected from donors asymptomatically infected with Plasmodium parasites. In the U.S., about one case of transfusion-transmitted malaria (TTM) is reported every other year. Although TTM is rare, it can be fatal.
Currently, the policy recommends rejecting blood donations from individuals who have been to malaria-prone zones until three months after their arrival back in the U.S. Individuals who have resided in areas endemic with malaria or who have received a malaria diagnosis are barred from donating blood for a duration of three years.
The imposition of these limitations was due to the absence of an approved diagnostic for evaluating blood donors. Previously, the FDA had advised a waiting period of one year before accepting donations from individuals who had visited malaria-endemic countries. This one-year guideline “led to a notable decrease of otherwise qualified donors,” according to the FDA in its 2022 guidance.
Although the FDA later took action to diminish the reduction of qualified donors, the present guideline continues to exclude individuals who are in good health from donating blood based on their travel experiences. Hypothetically, a diagnostic test for evaluating donor blood could allow a greater number of individuals to contribute.
Roche obtained approval for its diagnostic test following a study that included a group unable to donate blood due to their travel or residence in malaria-endemic regions. No infections emerged in the 159 samples. In the larger study involving more than 20,000 contributions from individuals with no known malaria exposure risk, all were found to be malaria-free.
The cobas Malaria test for use on the cobas 6800/8800 Systems (cobas Malaria) is a qualitative in vitro nucleic acid screening test for the direct detection of Plasmodium (P. falciparum, P. malariae, P. vivax, P. ovale and P. knowlesi) DNA and RNA in whole blood samples from individual human donors, including donors of whole blood and blood components, as well as other living donors. It is also intended for use in testing whole blood samples to screen organ and tissue donors when samples are obtained while the donor’s heart is still beating.

The firm established the test’s ability to identify malaria through another research conducted in a region of Nigeria where the parasite is widespread. In this trial, the test accurately identified malaria in 77 out of 199 symptom-free participants. An alternative nucleic acid diagnostic indicated 83 positive findings. Roche could not verify whether the discordant sample results were positive or negative.
The clinical specificity of cobas Malaria was evaluated on 20,187 individual blood donations collected at three different test sites (21.22% (4,284) from the American Red Cross, 47.24% (9,536) from Bloodworks Northwest, and 31.54% (6,367) from Gulf Coast Regional Blood Center). All 20,187 donations tested individually on cobas Malaria were non-reactive, demonstrating 100% clinical specificity (95% exact CI: 99.98%, 100.00%). No follow-up testing was performed on cobas Malaria non-reactive donations in this study.
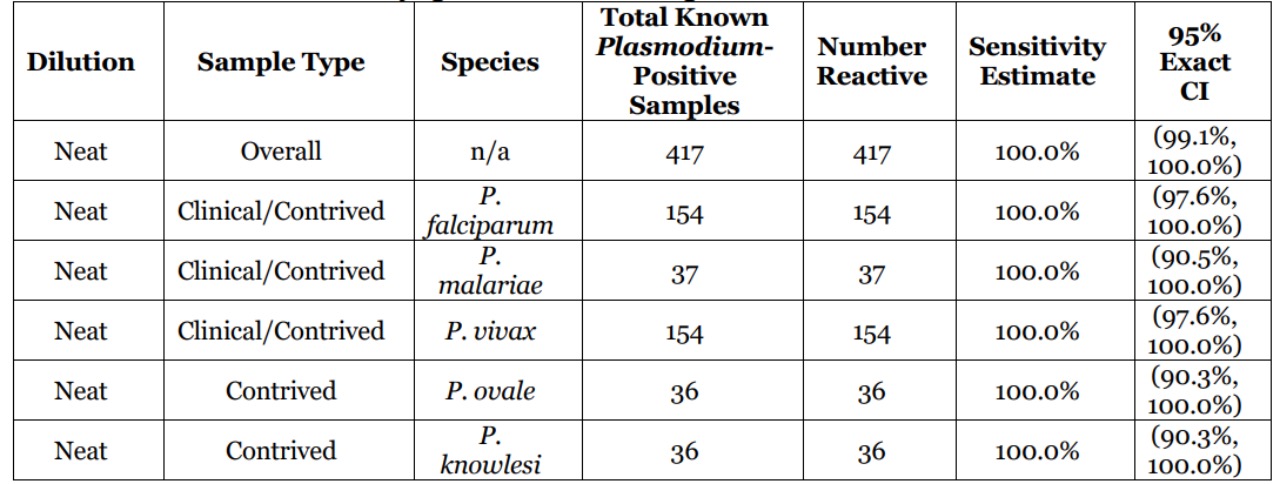
The clinical sensitivity of cobas Malaria was evaluated in a study testing 417 known Plasmodium-positive samples. These consisted of 237 clinical samples (118 P. falciparum, 118 P. vivax, and 1 P. malariae) and 180 contrived samples (36 spike samples:12 each at low, medium, and high concentration for each of the five Plasmodium species, falciparum, vivax, ovale, knowlesi, and malariae). All of the 417 neat samples were reactive with cobas Malaria resulting in a clinical sensitivity of 100% for overall (clinical and contrived combined) neat known Plasmodium-positive samples. The corresponding two-sided 95% exact CI for sensitivity was (99.1%, 100%).