Final – With a new $1.8 Million funding, Visby Medical Plans to Develop a Portable Molecular STI Tests
Published Mar. 09, 2024
By Hopkins Medtech
On February 9, 2024, the global nonprofit organization CARB-X (Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator) announced that it has allocated $1.8 million to Visby Medical for the advancement of a groundbreaking diagnostic tool. This funding will be used for the development of a portable molecular test designed to detect the pathogen responsible for gonorrhea and assess its susceptibility to the frontline antibiotic ciprofloxacin.
CARB-X’s initiative aims to address the urgent need for rapid and accurate diagnostics, particularly in regions with limited resources or difficult access to healthcare facilities. The diagnostic solution being developed by Visby Medical is expected to revolutionize disease detection and treatment decision-making.
Ms. Erin Duffy, Chief of Research and Development for CARB-X, emphasized the significance of Visby Medical’s portable PCR platform, highlighting its compact size and potential for rapid deployment. “Given the portability of the envisioned Visby Medical PCR platform, which fits in the palm of your hand, we see this as rapidly and highly deployable in low-resourced and hard-to-reach settings,” said Ms. Duffy.
Furthermore, the test holds the potential to effectively guide treatment strategies for gonorrhea patients. Mr. Gary Schoolnik, Chief Medical Officer for Visby, stressed the critical role of accurate and swift diagnostics in guiding treatment decisions. “Healthcare providers need accurate and rapid tests that identify the pathogen and its drug susceptibility,” commented Mr. Schoolnik.
The funding will not only support the development of a portable,instrument-free PCR-based diagnostic test but also aid in the creation of a multiplex test for gonorrhea, chlamydia, and trichomoniasis in urine samples from male patients. This expansion of capabilities underscores Visby Medical’s commitment to providing comprehensive solutions to combat sexually transmitted infections.
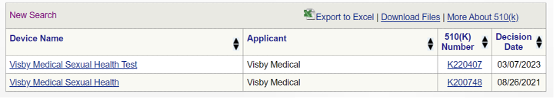
Visby Medical has already made significant strides in the field of diagnostics, with the US Food and Drug Administration granting 510(k) marketing clearance and a CLIA waiver for their existing test designed for female patients.
CARB-X affirmed its commitment to further supporting Visby Medical’s endeavors, with additional funding to be awarded upon the completion of project goals. The organization underscored Visby Medical’s intention to supplement these awards with funding from additional sources, ensuring sustained progress in test development and feasibility testing.
