The effects of aging are often associated with normal memory lapses, such as forgetting important dates, names, or favorite items. However, these incidents could also be early signs of Alzheimer’s disease (AD). With the increasing global prevalence of AD, it has become a major health issue, especially among the elderly. The urgency to improve AD diagnostic and treatment methods is emphasized by the fact that AD is the leading cause of dementia, accounting for more than 60% of all cases.
In brain neurons of Alzheimer’s disease patients, abnormal proteins such as beta-amyloid (Aβ) and tau protein accumulate together, forming plaques and neurofibrillary tangles, which are also widely recognized clinical characteristics.
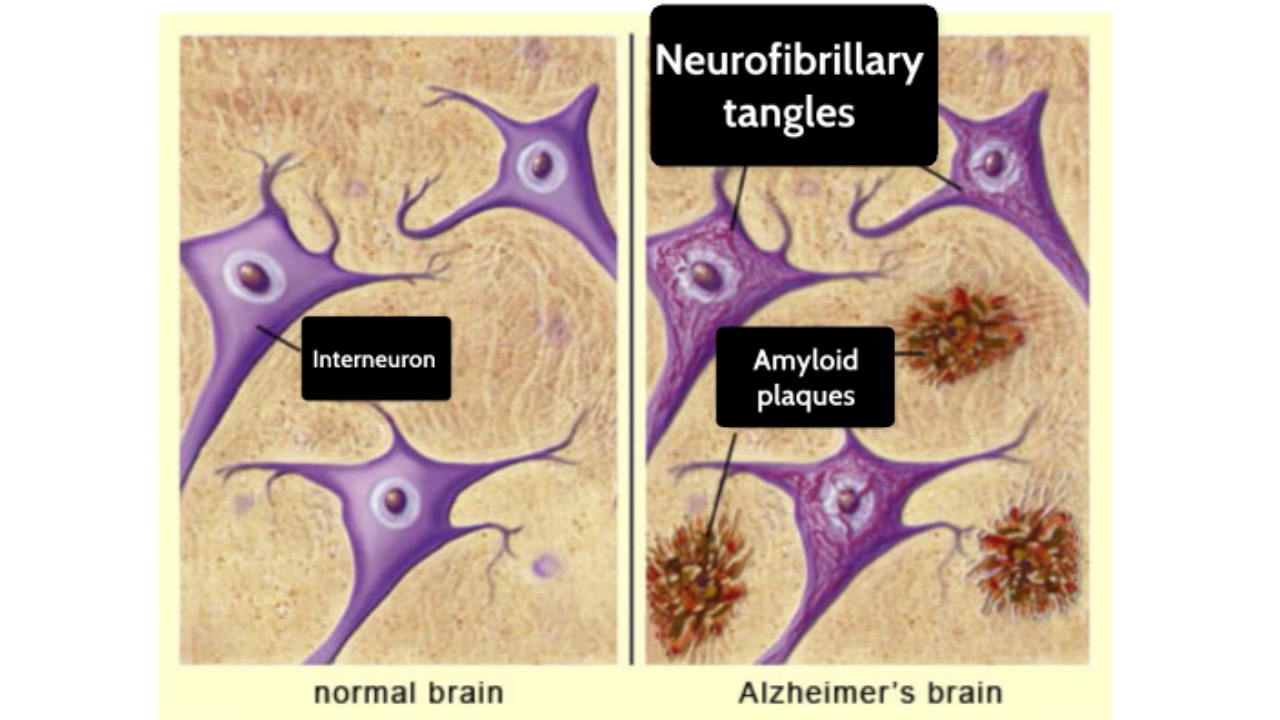
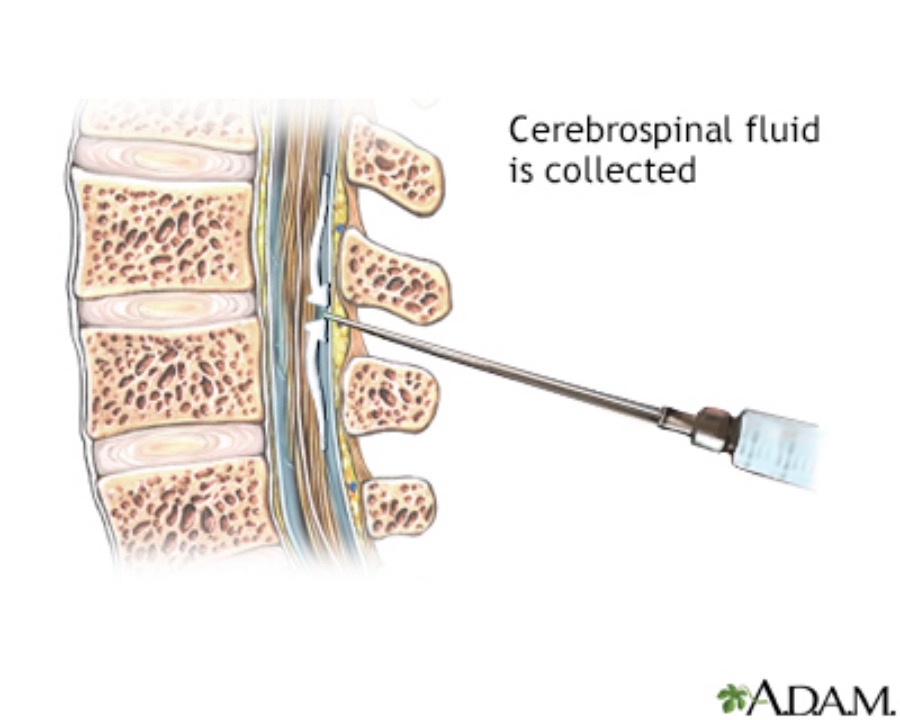
Several clinical researches show that the presence of amyloid-beta(Aβ)pathology is mainly confirmed by PET scans or cerebrospinal fluid testing. The shortcomings of these two methods are well known: they are costly, highly dependent on professionals, and even somewhat invasive.
Although still the gold standard, PET brain scans require a radioactive scan, has an average cost of $5,000 to $8,000 per scan. Another prevalent method involves analyzing amyloid-beta and tau protein levels in cerebrospinal fluid, which costs approximately $1,000 but involves a spinal tap process that some patients may find unappealing. On the other hand, blood test screening alone could be completed in less than six months and cut costs by tenfold or more.
Due to the risks and costs of puncture site infections, cerebrospinal fluid leakages, and nerve damages, it is not surprising to see patients preferring blood tests over PET scans or cerebrospinal fluid testing. Therefore, in clinical practice, these two testing methods are difficult to be widely promoted on a large scale.
Major companies have also introduced corresponding AD blood testing products. Quest has launched a direct-to-consumer AD-Detect Test, which measures the Amyloid Beta 42/40 Ratio; C2N Diagnostics offers PrecityAD2 for testing beta-amyloid; and Fujirebio currently only provides research-use plasma tests for the Aβ42/40 ratio and pTau 181. This trend indicates that blood testing is becoming a key method for AD screening.
Researchers from the Washington University School of Medicine in St. Louis and Lund University in Sweden have found that a blood test can detect molecular signs of Alzheimer’s disease in the brain as effectively as the FDA-approved cerebrospinal fluid (CSF) tests.
Interestingly, the team behind this research includes former employees of C2N Diagnostics, a company recognized as a the creator of the PrecivityAD blood tests for Alzheimer’s Disease.
The test uses mass spectrometry to compare the ratio of phosphorylated tau-217 proteins in the bloodstream to non-phosphorylated tau.
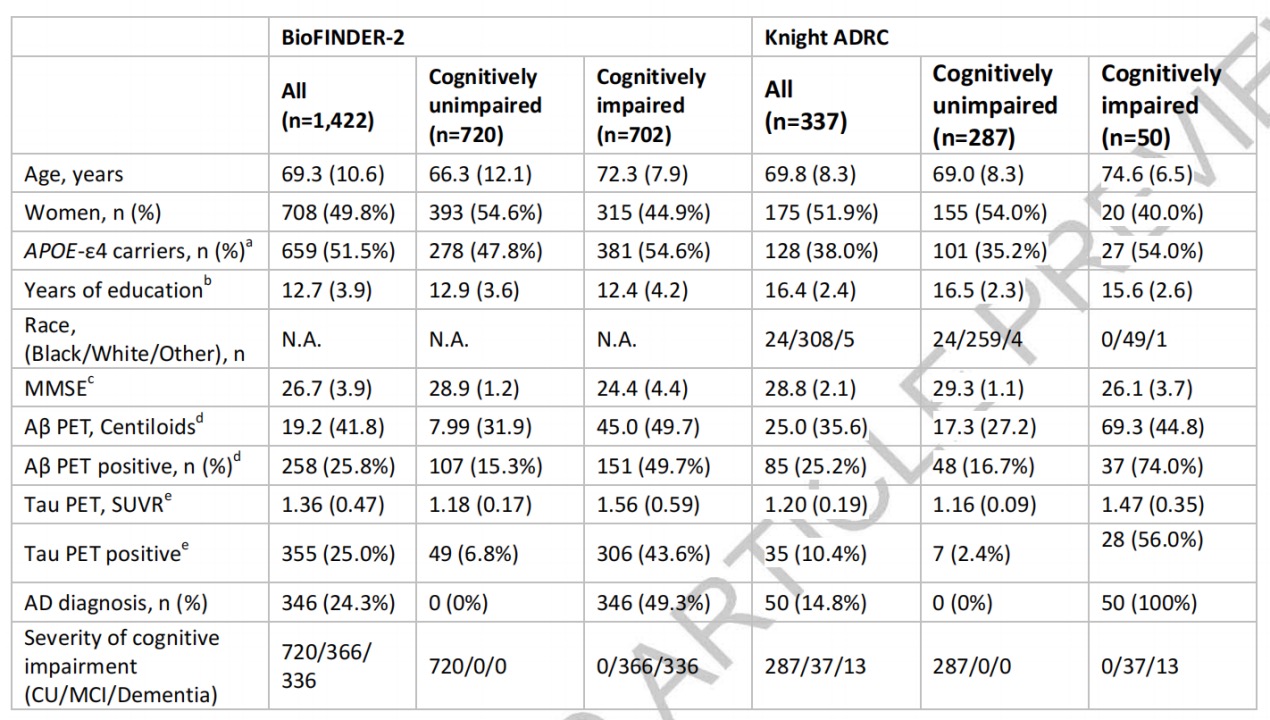
The team compared diagnostic performances of plasma %p-tau217 with the clinical use of FDA-approved CSF testing methods (Fujirebio’s CSF Aβ42/40 and Roche’s p-tau181/Aβ42) in independent cohorts in Sweden (BioFINDER-2 cohort) and the United States (Knight ADRC cohort).
Test results showed that the classification of Aβ PET status had high performance of plasma %p-tau217 (AUC=0.97) in the BioFINDER-2 cohort, comparable to CSF Elecsys p-tau181/Aβ42 (AUC=0.97) or CSF Elecsys Aβ42/40 (AUC=0.96). Similar results were obtained in the classification of Aβ PET status in the Knight ADRC cohort.
“We now have therapies that have clinical benefits, which is great, but they don’t reverse the loss of neurons in the brain,” Nicolas Barthélemy, the co-first author of the study published in Nature Medicine, said in a statement. “What we really want is to treat the disease before people start losing brain cells and showing symptoms.
Recognizing individuals with Alzheimer’s disease has become crucially significant, particularly with the recent introduction of the first treatments capable of slowing the disease’s progression to patients, along with other promising drugs in development. These treatments may be more successful if administered earlier in the disease’s course, highlighting the importance of early detection.
Moving forward, this research has implications of revolutionizing Alzheimer’s diagnosis, as blood tests are less invasive and potentially more accessible than spinal fluid exams. Recent developments show potential in earlier and more widespread detection of Alzheimer’s Disease, where they enable timely interventions and enhance the efficiency of clinical trials for new treatments. All of these signifies a glimmer of hope for those impacted by Alzheimer’s disease.