Acon’s at-home COVID antigen test kit has received the first FDA 510(k) certification
Published Mar. 01, 2024
By Hopkins Medtech
November 9th, the FDA announced that Acon’s Flowflex COVID-19 Antigen Home Test became the first over-the-counter (OTC) COVID antigen test to receive 510(k) clearance, with a sensitivity of 89.8% and specificity of 99.3%. This policy signifies that future OTC COVID antigen test kits will follow the traditional channel.
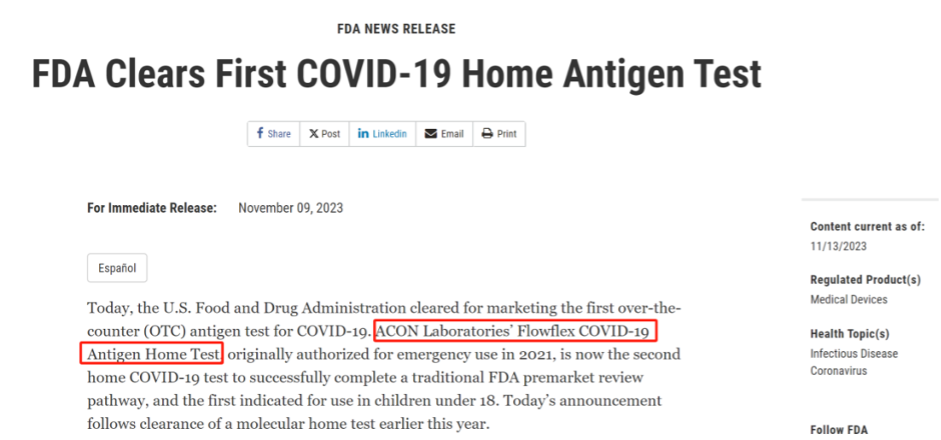
Developed by Acon, the Flowflex COVID-19 Antigen Home Test initially received Emergency Use Authorization (EUA) in 2021. It is the second home COVID test product to successfully pass through the FDA’s traditional market pathway after Cue Health announced the FDA’s De Novo approval for its at-home molecular test in June this year.
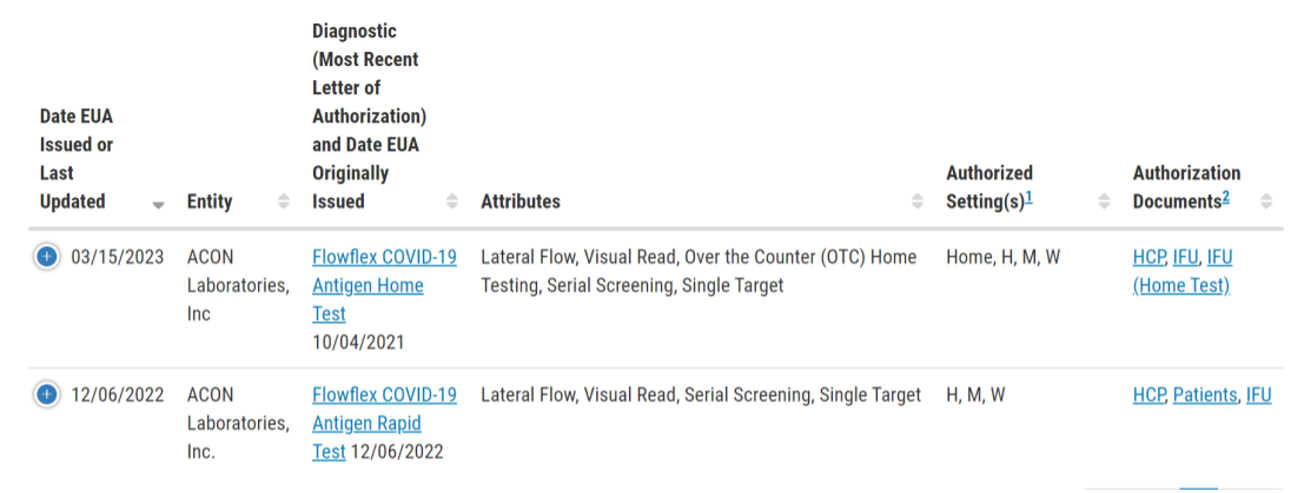
EUA was designed to enable the FDA to swiftly provide COVID testing in the United States during public health emergencies. Currently, there are 65 antigen and 276 molecular COVID tests with EUA, including 37 OTC testing products. While EUA remains effective, the FDA is encouraging manufacturers to transition to traditional application pathways, as outlined in guidance issued in March this year, once the emergency ends.
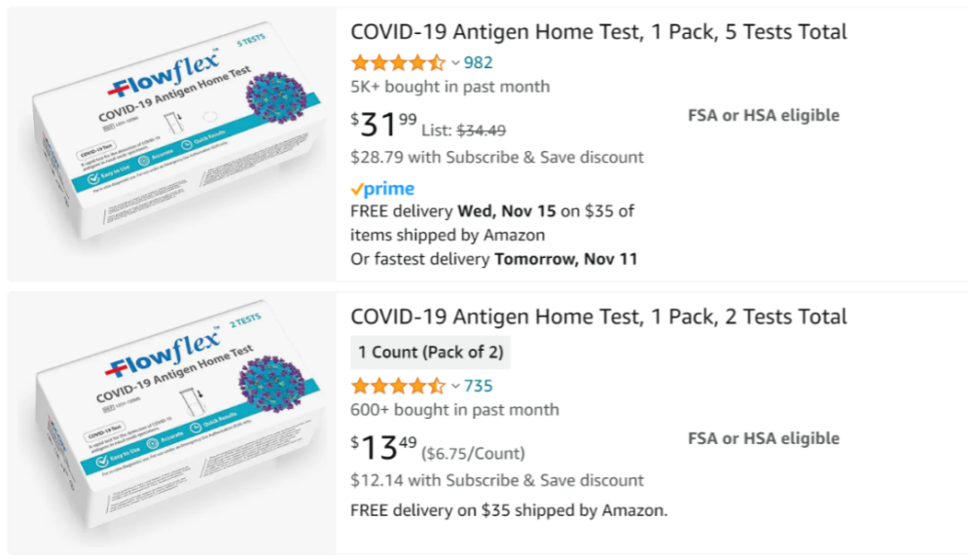
Acon has established a substantial presence in US retail channels, with the Flowflex COVID-19 Antigen Home Test available at high-volume stores like Costco, ubiquitous pharmacies like Walgreens, and popular online retailer Amazon. According to our long-term tracking, on Amazon’s platform, individual Flowflex tests have been consistently priced between $6.4 and $6.9.
Based on Amazon’s latest data, purchases of the Flowflex COVID-19 Antigen Home Test (5-pack) have exceeded 5,000 units in the past month, indicating strong sales and acceptance among the US populace.
Despite the FDA opening traditional channels for COVID testing products, several publicly traded companies’ financial reports clearly indicate a decline in demand. For instance, Siemens Healthineers stated that COVID testing wouldn’t contribute to the company’s revenue in the coming year. COVID testing is expected to become a standard part of annual fall and winter diagnostics but might shift towards multi-panel testing instead of standalone COVID tests. The FDA’s approval of the first 510(k) COVID antigen test paves the way for companies to apply for respiratory disease multi-panel testing!



