Significant Gap in EU Medical Device Market Revealed, the MDCG’s Prompting Calls for Immediate Certification Applications
Published Feb. 26, 2024
By Hopkins Medtech
The EU released a market report at the end of the previous month, which revealed a substantial gap in the European Union medical device market. As per the In Vitro Diagnostic Directive (IVDD), only an estimated 8% of In Vitro Diagnostics (IVDs) necessitate the involvement of a notified body for conformity assessment. However, during the In Vitro Diagnostic Regulation (IVDR) period, this fraction dramatically surged to 80%.
Moreover, as of June this year, a mere 1,150 IVDR applications were registered, with only 500 certificates issued.
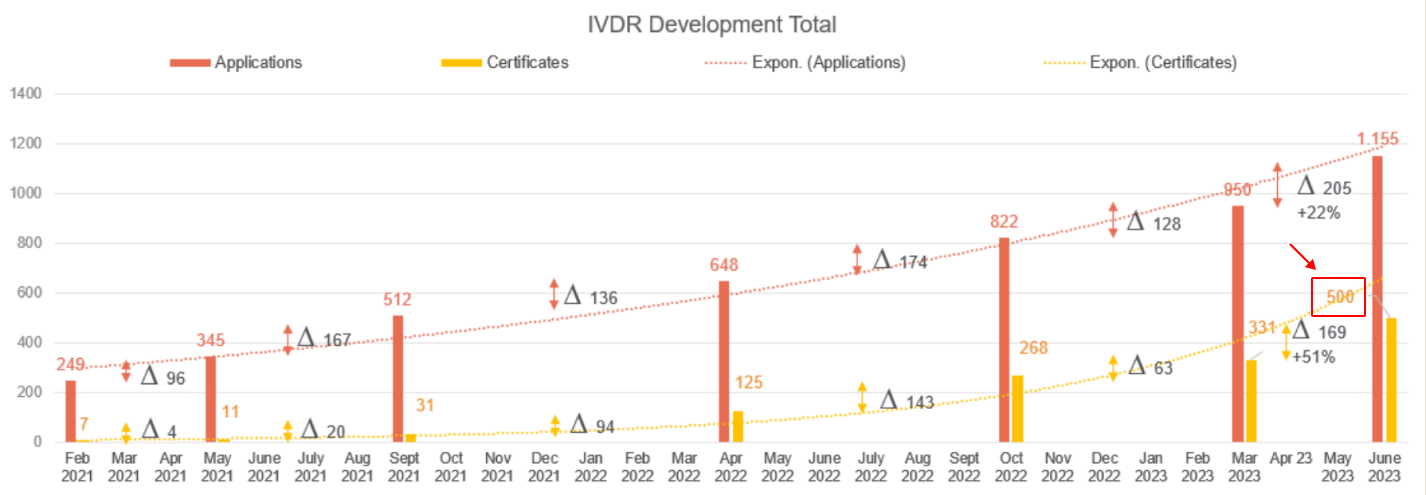
In response to this report, the Medical Device Coordination Group (MDCG) encouraged manufacturers to promptly submit applications for certification. At present, the review process for products by notified bodies is experiencing delays due to potentially incomplete application materials, a stark contrast from the prior IVDD periods. The MDCG especially urged manufacturers of IVDR Class D products, set to expire in 2025, to expedite the process. For ongoing monitoring of IVDR progress, manufacturers are being asked by the MDCG to regularly provide updates on the status of their devices.
Simultaneously, the MDCG panel urged notified bodies to streamline the certification process and consider the application fees for Small and Medium-sized Enterprises (SMEs). The panel highlighted the importance of structured communication between notified bodies and manufacturers to ensure effective regulatory guidance and technical support. To keep track of IVDR progress, the MDC
