CareDx has its monitoring tool for lung transplant rejection covered by CMS
Published Feb. 25, 2024
By Hopkins Medtech
CareDx recently announced that its AlloSure Lung, a monitoring tool for lung transplant rejection reactions, has gained coverage under the US Centers for Medicare and Medicaid Services (CMS) MolDx program. As of May 9th, the test will be used for monitoring lung transplant patients in the local coverage determination (LCD) for molecular testing of solid organ transplant rejection reactions.
AlloSure Lung was introduced in 2021 and has been validated to identify acute cellular rejection and antibody-mediated rejection and infections in asymptomatic lung transplant recipients.
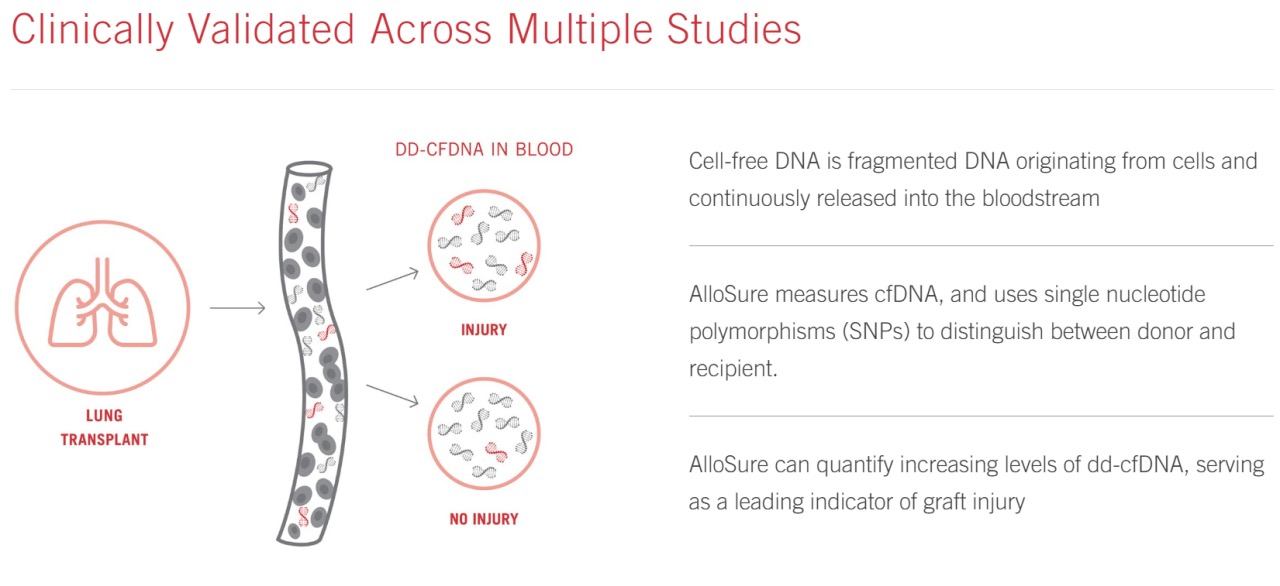
This CMS coverage for AlloSure Lung may potentially lead to improved performance for CareDx. However, this is not the first time CareDx has obtained CMS coverage. Since 2020, they have had medical insurance coverage for their AlloSure donor-derived cell-free DNA test for kidney and heart transplant patients.
Despite currently being in a deficit state, CareDx’s latest financial performance for the first quarter of 2023 showed record numbers of patient testing results for AlloMap and AlloSure, totaling approximately 49,900 tests, representing a 17% year-over-year growth.
Interestingly, CareDx recently had a dispute with Natera that has now been resolved. The US District Court for Delaware overturned a previous ruling from March 2022 in CareDx’s lawsuit against Natera. In the 2019 lawsuit, CareDx claimed that Natera’s advertising was misleading and made potential customers believe that Natera’s kidney transplant rejection detection product, Prospera, was superior to CareDx’s AlloSure. This is not the first time CareDx has sued Natera, as they previously sued Natera for patent infringement, with CareDx ultimately losing the case. This illustrates the fierce competition that CareDx faces despite its focus on organ transplant services.
CareDx was founded in 1998 and is headquartered in California. It is a leading precision medicine solutions company, specializing in developing and selling clinically differentiated,high-value medical solutions for transplant patients and healthcare providers. CareDx provides testing services, products, and digital solutions for pre- and post-transplant patients, as well as relevant genomic information for transplant recipients.
According to data from Blue Weave Consulting, the transplant diagnostics market was valued at approximately $3.9 billion in 2021 and is expected to reach $6.4 billion by 2028, with a CAGR of 7.4% during the forecast period. In the US, there are over 40,000 organ transplant surgeries performed each year, with the most common organs being the kidneys, liver, heart, and lungs. Apart from CareDx, other companies involved in the transplant diagnostics field include Immucor Transplant and GenDx.

