Danaher (stock code: DHR.N) announced on Tuesday that it will provide Cepheid’s Xpert MTB/RIF Ultra tuberculosis testing products to the Global Fund at cost price, which is $7.97. The company ensures the distribution of millions of tests each year in underdeveloped countries. Danaher states that $7.97 is the cost price, and the group will not make a profit. The company has also committed to allowing third-party organizations to annually verify their costs.
Peter Sands, the Executive Director of the Global Fund, stated that this price reduction will enable people in need worldwide to access an additional 5 million tests.
However, according to Forbes, Danaher’s price reduction was influenced by pressure from international health and aid organizations such as the Global Fund, the Stop TB Partnership, and USAID. These organizations initially lobbied the company to lower the price by 50% to around $5, but Cepheid’s initial price was $16.86, already 75% lower than the market price. Tuesday’s announcement to reduce it to $7.97 ultimately reached a consensus.
Cepheid’s Xpert MTB/RIF Assay was developed in collaboration with FIND and the Bill and Melinda Gates Foundation. The product utilizes Cepheid’s GeneXpert platform and is a qualitative, nested real-time PCR test for detecting Mycobacterium tuberculosis complex DNA in sputum or concentrated sputum deposits. The product received FDA 510(k) clearance in February 2015 (510(k) number: K143302). The primary target population for the Xpert MTB/RIF Assay is patients with suspected active pulmonary tuberculosis, i.e., those already exhibiting symptoms. Additionally, the product can test positive patients for resistance to rifampin, helping physicians tailor appropriate treatment regimens.

The Global Fund is an international non-profit organization established in 2002, headquartered in Geneva, Switzerland. Its primary mission is to provide funding and resource support for the global fight against major infectious diseases such as HIV/AIDS, tuberculosis, and malaria. The Global Fund’s goal is to reduce the transmission of these diseases in affected countries and regions, save lives, improve public health, and enhance the sustainability of health systems.
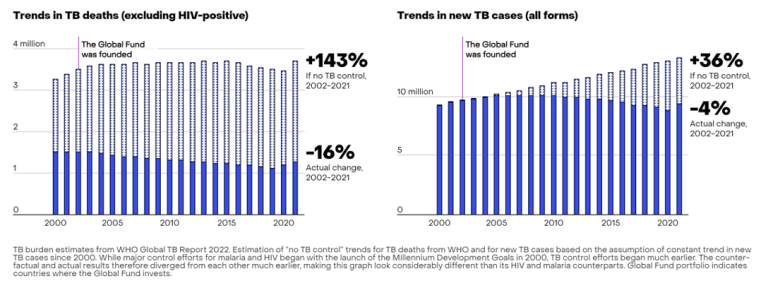
According to the Global Fund, as of June 2023, it has provided 76% of international funding for tuberculosis and invested $9.2 billion in projects for the prevention and treatment of tuberculosis. It has also invested $1.5 billion in tuberculosis/HIV programs. In countries where the Global Fund has made investments between 2002 and 2021, tuberculosis-related deaths have decreased by 16%. Without these interventions, tuberculosis-related deaths during the same period would have increased by 143%.
Regarding TB testing, it is a relatively popular diagnostic test, but there are different types of tests for TB. In addition to tests for patients with symptoms, such as the Xpert MTB/RIF Assay, there are tests for latent Mycobacterium tuberculosis. Typically, these tests use methods like skin tests and IGRA (Interferon-gamma release assay) tests. More accurate IGRA tests have gradually replaced traditional skin tests.
In the United States, only two TB tests, Qiagen/DiaSorin’s QuantiFERON-TB and Oxford Diagnostics’ T-SPOT.TB Test, have received FDA clearance. Both tests use highly accurate blood test methods. The QuantiFERON-TB test is estimated to cost about $70 per person.
In the latest Q2 2023 earnings report, Qiagen mentioned that its TB testing product, QuantiFERON-TB testing, generated $104 million in global revenue, a 27% increase from the same period the previous year, with the highest proportion of testing in the United States. QuantiFERON-TB is also the product with the highest market share in the United States.
In 2022, the United States reported 8,300 cases of tuberculosis, compared to 7,874 cases in 2021. The incidence of tuberculosis increased slightly in 2022, from 2.4 per 100,000 in 2021 to 2.5 per 100,000. CNN reports that this level has returned to pre-pandemic levels.
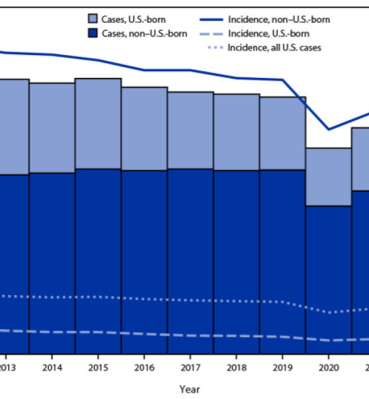
Despite this, there is a significant population in the United States that requires testing, including:
Immigrants: TB blood testing is mandatory for immigrants to the United States, and approximately one million people immigrate to the United States each year.
Healthcare Workers: Due to the higher risk of infection for healthcare workers, they are also required to be tested. There are approximately 22 million healthcare workers in the United States.
Prison Populations: Prisons, being closed environments with a higher prevalence of TB, are more susceptible to Mycobacterium tuberculosis transmission. Thus, TB testing is mandatory in prisons.