Sebia Gains FDA 510(k) Clearance for Hemoglobin Disorder Analyzer
Sebia, a global specialty diagnostic company providing innovative solutions for screening and diagnostics in oncology, metabolic diseases, genetic disorders, and autoimmune diseases, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for CAPILLARYS 3 DBS devices.

The company’s Capillarys 3 DBS instrument is used for the qualitative detection by electrophoresisof normal hemoglobins F and A and abnormal hemoglobins S, C, E, D, and Bart’s in a dried bloodspot sample that is collected on filter paper. The firm said that the instrument provides high-throughput and full traceability from sample to results.
A hemoglobin disorder or hemoglobinopathy is an inherited blood disorder in which there is an abnormal form of hemoglobin (variant) or decreased production of hemoglobin (thalassemia). A hemoglobinopathy evaluation is a group of tests that determines the presence and relative amounts of abnormal forms of hemoglobin in order to screen for and/or diagnose a hemoglobin disorder.
Hemoglobin (Hb) is the protein in red blood cells (RBCs) that binds to oxygen in the lungs and allows RBCs to carry the oxygen throughout the body, delivering it to the body’s cells and tissues. Hemoglobin consists of one portion called heme, which is the molecule with iron at the center, and another portion made up of four globin (protein) chains. Depending on their structure, the globin chains, depending on their structure, have different designations: alpha, beta, gamma, and delta. The types of globin chains that are present are important in the function of hemoglobin and its ability to transport oxygen.
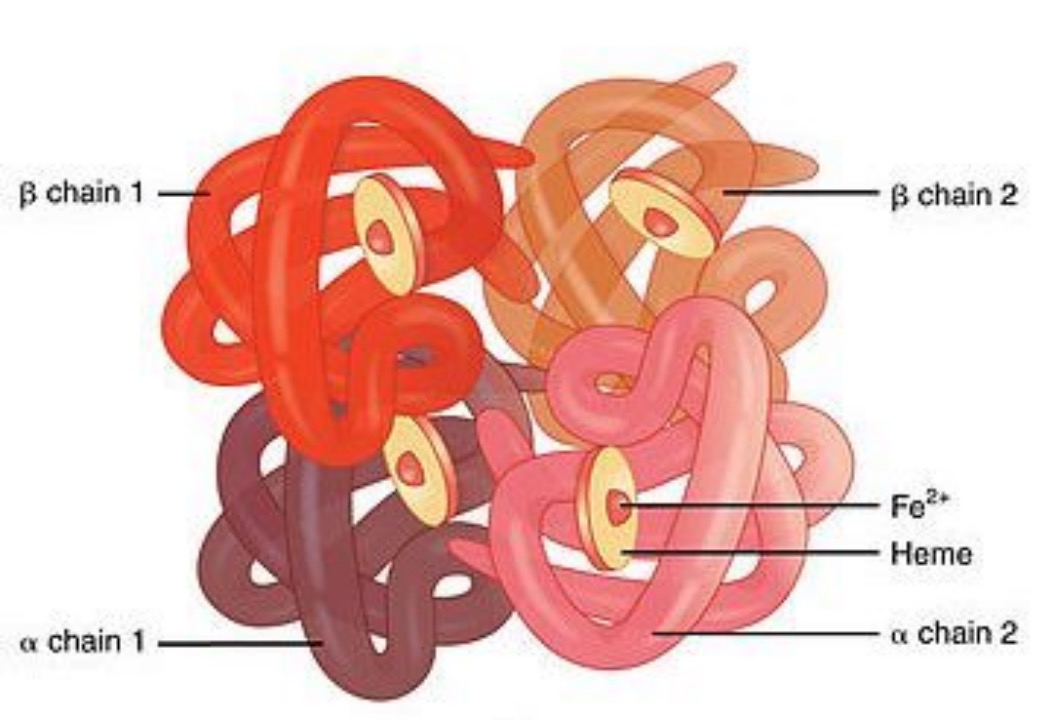
Normal hemoglobin types include:
Hemoglobin A: makes up about 95%-98% of Hb found in adults; it contains two alpha and two beta protein chains.
Hemoglobin A2: makes up about 2%-3% of Hb in adults; it has two alpha and two delta protein chains.
Hemoglobin F (fetal hemoglobin): makes up to 1%-2% of Hb found in adults; it has two alpha and two gamma protein chains. This is the primary hemoglobin produced by the fetus during pregnancy; its production usually falls shortly after birth and reaches adult levels by 1-2 years.
Sebia’s solution is intended for the detection of normal hemoglobins (F and A) and abnormal hemoglobins (S, C, E, D, and Bart’s) in blood from human newborn collected on filter paper. This qualitative analysis is performed by capillary electrophoresis with the CAPILLARYS 3 DBS automated instrument. This offer provides laboratories a gain in operational efficiency with high throughput and full traceability from DBS card up to the result through a cyber-secured environment.
ABOUT Sebia
Founded in 1967, Sebia is a world-leading provider of clinical protein electrophoresis equipment and reagents, a technology used for in vitro diagnostic testing. Its systems analyze proteins in order to screen and monitor various diseases and conditions; primarily oncology (multiple myeloma) and metabolic disorders such as diabetes, also hemoglobinopathy and rare pathologies.
Following the acquisition of Orgentec, Corgenix, Arotec in 2021, and of Zeus Scientific in 2022, Sebia now develops and markets innovative solutions for autoimmunity diagnostics.
Headquartered in Lisses, France, the company operates across more than 120 countries. Sebia is owned by CVC Capital Partners, Tethys Invest, and the Caisse de Dépôt et Placement du Quebec (CDPQ).
https://www.sebia.com