Oxford Nanopore collaborates with Day Zero Diagnostics to develop an instant sepsis diagnosis solution
Published Mar. 06, 2024
By Hopkins Medtech
This Tuesday, Oxford Nanopore, a nanopore sequencing company, partnered with infectious disease diagnostics firm Day Zero Diagnostics to develop an end-to-end diagnostic solution for blood infections—sepsis.
The collaboration aims to integrate and optimize clinical diagnostic systems for automated sample processing and whole-genome sequencing, allowing same-day identification and genome-based antibiotic susceptibility analysis without the need for blood culture. The planned diagnostic system will combine Day Zero’s highly enriched sample preparation technology and its AI-driven Keynome microbial identification and antibiotic sensitivity pipeline with Oxford Nanopore’s high-throughput PromethIon 2 Solo sequencing reads.
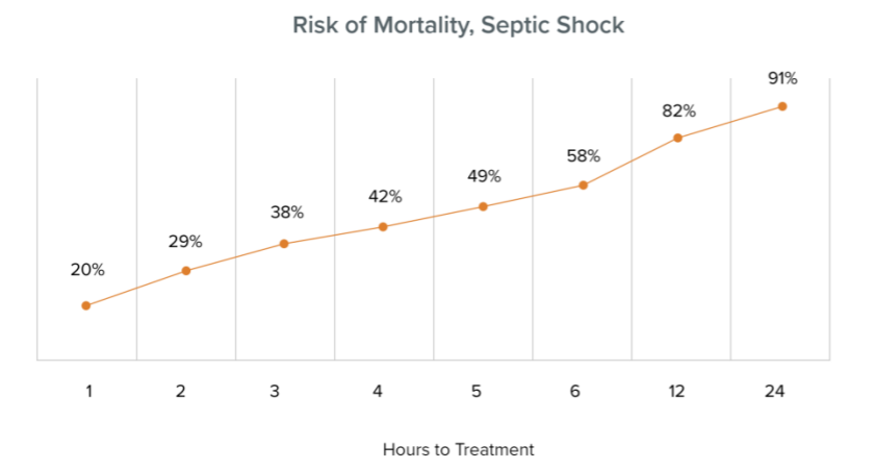
According to WHO data, there are approximately 11 million sepsis-related deaths annually worldwide, accounting for one-fifth of global deaths. In the United States, it affects 1.7 million adults annually and is a leading cause of death in one-third of US hospitals. In severe infections like sepsis, the risk of death increases over time, making early and effective treatment crucial. However, current culture-based diagnostics, including antibiotic sensitivity assessments, might take several days to yield results.
Day Zero Diagnostics’ CEO, Jong Lee, mentioned that direct culture-free organism identification and antimicrobial susceptibility analysis from local samples on the same day could be game-changing, enabling earlier and more targeted treatment for patients.
In August this year, the CDC launched the Hospital Sepsis Program Core Elements, offering a free resource center for hospitals to initiate or enhance their facilities’ identification and management of sepsis.
Regarding sepsis diagnostics, the FDA approved IntelliSep by Cytovale last December, the first sepsis diagnostic product for use in emergency departments. It evaluates cell host response via white blood cell biophysical characteristics, used in both clinical assessment and laboratory settings. It can identify a patient’s risk of sepsis simply and clearly and provides results within 10 minutes to aid in early sepsis detection.
Additionally, T2 Biosystems, another player in sepsis diagnostics, introduced the T2Dx Instrument,T2Bacteria Panel (510(k) number:K172708),and T2Candida Panel (510(k) number:K173536). According to T2’s 2022 annual report, the sepsis-related revenue for the year reached $8.4 million, showing a 17% increase from 2021, with Q4 of last year setting a revenue record in the United States. T2 Biosystems secured 51 placements of T2Dx Instruments throughout the year, with 27 of them in the US.
Apart from their collaboration on diagnostic platforms, Day Zero Diagnostics also signed an agreement to use Oxford Nanopore’s platform for sequencing and analysis, forming part of their epiXact laboratory services offered to commercial customers.
Based in Boston, Day Zero Diagnostics is a diagnostics company specializing in microbial infections. They leverage whole-genome sequencing and artificial intelligence to combat the rise of antibiotic-resistant infections. Earlier in August this year, Day Zero received a Phase I Small Business Innovation Research (SBIR) grant from the NIH’s National Institute of Allergy and Infectious Diseases (NIAID), funding the development of epiXact FMT, a drug vigilance test aimed at enhancing the safety and effectiveness of fecal microbiota transplantation (FMT) clinical trials.


