Natera’s transplant testing assay, Prospera Lung, meets the coverage requirements of CMS, signaling the increasing maturation of the transplant monitoring market
Published Mar. 06, 2024
By Hopkins Medtech

This week, Natera (Stock code: NTRA.O) announced that its Prospera Lung donor-derived cell-free DNA (dd-cfDNA) transplant assessment test has met the coverage requirements of the US Centers for Medicare and Medicaid Services (CMS) Molecular Diagnostic Services Program (MolDX). The clinical validation of Prospera Lung (VALID) was published in 2022, emphasizing its performance with 195 matched biopsy samples from 103 patients. This prospective study analyzed the relationship between Prospera Lung and various complications of lung transplantation, including acute rejection (AR), chronic lung allograft dysfunction (CLAD), and allograft infections. The study demonstrated an area under the curve (AUC) of 0.91 when analyzing AR in stable patients.
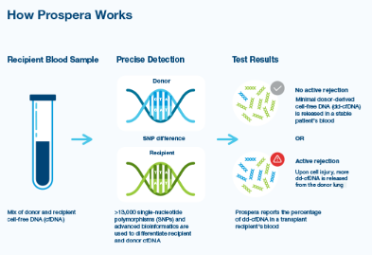
The Prospera test utilizes Natera’s massively multiplexed PCR (mmPCR) technology based on single-nucleotide polymorphisms (SNPs) to non-invasively, accurately, and precisely identify allograft rejection without the need for prior donor or recipient genotyping. The test measures the proportion of donor-derived cell-free DNA (dd-cfDNA) in the recipient’s bloodstream. It can be used by physicians when considering a diagnosis of active rejection and helps exclude this scenario when evaluating the necessity of diagnostic testing or the results of invasive biopsies. It already received CMS coverage (Code: A57980) for kidney transplant monitoring in 2021.
Last month, CareDx’s AlloSure Lung for monitoring lung transplant rejection also received coverage under the MolDx program.
The five-year survival rate for lung transplants is 59%, the lowest among all organ transplants. The improvement in recipient survival has been limited over the past 20 years, primarily due to acute rejection (AR) and chronic lung allograft dysfunction (CLAD), which are the leading causes of lung transplant recipient mortality.
Two weeks ago, Natera also released its Q2 2023 financial report, indicating a robust business performance. The company reported revenue of $261 million, a 32% YoY growth. In this quarter, they processed around 617,200 tests, a 23.5% increase compared to approximately 499,900 tests processed in the same period last year. Tumor tests were conducted 83,500 times in this quarter, an 88.9% increase from around 44,200 tests conducted in the same period last year.
Natera achieved a favorable outcome in the lawsuit against ArcherDX/Invitae, resulting in a compensation of $19.35 million for lost profits and royalty fees from past sales, and confirming the validity of all three Natera patents. In the lawsuit against CareDx, the court overturned the jury’s verdict, relieving Natera of a $45 million compensation.
Following the announcement of the financial report, Natera’s stock price rose by 17.6%.
According to data from Blue Weave Consulting, the transplant diagnostics market was valued at approximately $3.9 billion in 2021 and is projected to reach $6.4 billion by 2028, with a CAGR of 7.4%. In the United States, there are over 40,000 organ transplant surgeries conducted annually, with kidney, liver, heart, and lung transplants being the most common types.

