Tempus Labs Optimistic on MRD, Invests in and Assists Personalis in Commercialization
Published Mar. 06, 2024
By Hopkins Medtech
Precision oncology genomics company, Personalis, and AI and precision medicine company, Tempus Labs, have announced a strategic collaboration to jointly commercialize NeXT Personal Dx, a test developed by Personalis’ whole-genome liquid biopsy laboratory for detecting minimal residual disease (MRD) and recurrence in lung and breast cancers.
Under this agreement, Tempus will incorporate NeXT Personal Dx, the whole-genome liquid biopsy analysis, into its test menu and promote it to oncologists. Additionally, Tempus will make milestone payments of up to $12 million to support clinical evidence development for the laboratory-developed test (LDT) of MRD. Personalis will also provide compensation to Tempus based on the market value of sales, marketing, order submissions, and result delivery.
Furthermore, Personalis will grant Tempus warrants to purchase up to approximately 9.2 million shares of Personalis common stock within the next 24 months.
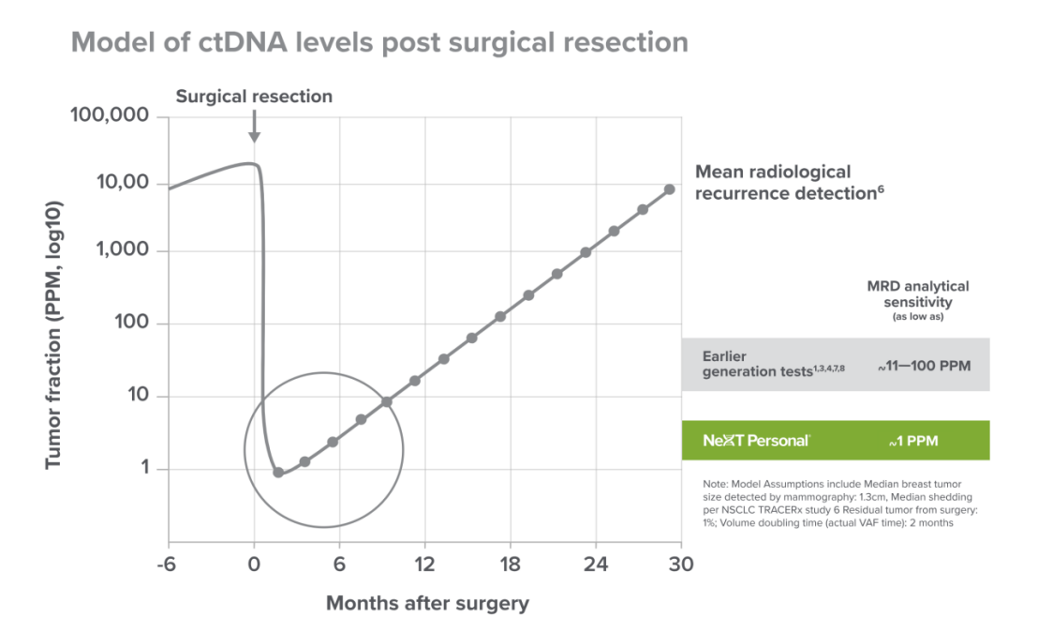
Next Personal Dx is an LDT test introduced by Personalis a month ago for comprehensive whole-genome liquid biopsy testing. Its analysis sensitivity reaches as low as ~1PPM (parts per million) or 1×10-6 tumor fraction, with a specificity of over 99.9%. The test utilizes Personalis’ proprietary NeXT SENSE technology for signal enhancement and noise suppression, providing highly sensitive detection when combined with personalized tumor characteristics.
Founded in 2015, Tempus Labs is a technology-driven healthcare company focused on fundamentally transforming cancer care through data analytics, molecular diagnostics, and precision medicine. Tempus collects and analyzes clinical and molecular data using cutting-edge technology to offer personalized insights and treatment choices for cancer patients. In October last year, Tempus secured a funding of $275 million, totaling approximately $1.3 billion in funding.
