Despite being entangled in lawsuits, Genoscopy ventures into the inflammatory bowel disease detection field to break through early screening for colorectal cancer
Published Mar. 02, 2024
By Hopkins Medtech
Colorectal cancer early screening company Genoscopy and AdisoTherapeutics announced a strategic collaboration this Tuesday. The two parties will utilize Genoscopy’s transcriptome platform from fecal samples to evaluate patients’ responses to Adiso’s experimental treatment for inflammatory bowel disease.
Genoscopy will prospectively assess gene expression changes in fecal samples of patients involved in Adiso’s ADS051 Phase II clinical trial. ADS051 by Adiso is an orally administered,intestine-restricted small molecule modulator of neutrophil transport and activation. Genoscopy’s platform will be used to identify potential responders to therapy and potentially monitor clinical remission minimally invasively.
Moreover, the clinical recruitment for the global second-stage trial is set to commence next year. Adios also plans to leverage Genoscopy’s platform in future trials to assess mucosal healing and predict patient responses to treatment.
Recent news about Genoscopy has been frequent. Initially, on November 14th, it signed a multi-year collaboration agreement with Labcorp, the largest clinical laboratory in the United States, to jointly promote Genoscopy’s colorectal cancer early screening test, ColoSense. Currently, Genoscopy has applied to the FDA in January of this year, and the FDA is in the process of reviewing it. Once FDA-approved, LabCorp will offer the test. Genoscopy will conduct this test, enabling healthcare clients to conveniently order through Labcorp as part of their comprehensive screening programs.
However, the good times were short-lived. On November 17th, leading colorectal cancer early screening company Exact Sciences filed a patent infringement lawsuit against Genoscopy in the Delaware District Court. The lawsuit alleges that Genoscopy’s ColoSense colon and rectal cancer screening test infringed on Exact Sciences’ U.S. Patent 11634781 for Cologuard. Exact Sciences also claims in its complaint that ColoSense utilizes the method covered by patent 781 on fecal sample handling without permission from Exact Sciences. If ColoSense receives FDA approval, it could potentially claim Exact Sciences’ current market share. Therefore, Exact Sciences is seeking an injunction against the manufacturing, use, sale, or importation of ColoSense into the United States, along with compensation for alleged infringement losses.
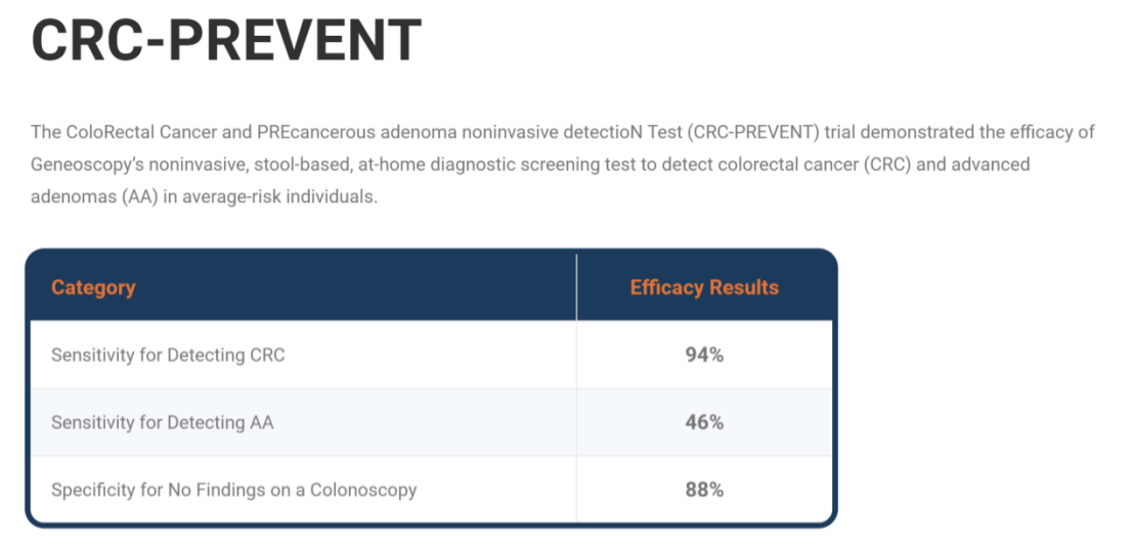
Genoscopy published the results of a prospective clinical study CRC-PREVENT in the Journal of the American Medical Association. The clinical trial recruited a total of 8,920 individuals aged 45 and above between June 2021 and June 2022, with an average age of 55 in the final cohort. Overall, ColoSense demonstrated a sensitivity of 94% for colorectal cancer screening and a sensitivity of 46% for advanced adenomas.
Compared to the currently available Cologuard, ColoSense showed better sensitivity for cancer and advanced precancerous lesions (Cologuard was 92% and 42%, respectively), but its specificity was lower than Cologuard’s 90%.
Additionally, ColoSense maintained high accuracy in individuals under 50, which Exact Sciences has not yet demonstrated. Genoscopy’s unique approach combines gene expression (assessed via ddPCR) with fecal immunohistochemistry tests and clinical evaluation of smoking status to generate final risk results.
Furthermore, Genoscopy mentioned its recruitment strategy with DeeP-C, and other studies differed. Researchers did not direct patients to conventional colonoscopy clinics for routine endoscopic examinations. Instead, they used social media to seek participants within specific age groups for screening and provided monetary incentives to drive compliance. Specifically, participants received a $200 participation fee, with $50 awarded to those who provided fecal samples, and the remaining $150 provided after completion of colonoscopy.
Previously mentioned, despite colorectal cancer early screening being the most widespread among various cancer screenings, the testing market is far from saturation. According to Statista data, approximately 19.79 million individuals in the 45-49 age group in the United States in 2021, assuming around 70% of the population undergoes colorectal cancer screening (currently, the screening rate for the 50-75 age group in the U.S. is 70%), leading to approximately 13.85 million additional individuals undergoing colorectal cancer screening.
Today, more and more companies are joining the battlefield of early colorectal cancer screening. Apart from Exact Sciences and Genoscopy, other companies like Guardant Health, Freenome, DiaCarta, Mainz Biomed, etc., are eagerly vying for market share. As competition intensifies, the ultimate beneficiaries will be the American population, providing them with more choices.
