Securing $70 million from Quest, let’s welcome the next challenger to Cologuard
Published Feb. 20, 2024
By Hopkins Medtech
Since the launch of Cologuard in 2014, it has become the towering peak and longest river in the field of early screening for colorectal cancer. Benchmarking against Cologuard has become a habit as if one cannot prove oneself without surpassing it.

Of course, Exact Sciences, sitting alone at the summit, has not rested on its laurels. It has been closely monitoring the challengers attempting to challenge its position. On November 17, Exact Sciences filed a patent infringement lawsuit against Geneoscopy. Whether it is a real infringement or not, who cares? Let’s see first and see. Poor Geneoscopy had just secured a distribution agreement with Labcorp, and before they could celebrate for two days, they found themselves in a tight spot again.

While the battle between Exact Sciences and Geneoscopy is heating up, Quest is not sitting idle either. Enter Universal DX (UDX), “Here’s 70 million; let me have a slice of the early screening cake too,” says Quest. Quest: “The early screening cake smells good, give me a piece too.” UDX: “I only do blood tests and LDT; you wouldn’t sue me too, would you?”

Setting Geneoscopy aside for now, what’s the deal with UDX? On November 21, Quest announced that it would acquire the copyright for UDX’s Signal-C test in the United States for $70 million, with clinical testing restricted to Quest’s clinical sites in Texas.

Okay, the dominance and possessiveness are practically overflowing between the lines. Quest indeed has the credentials to be a boss, with a network spanning 2,100 service centers across the U.S., serving one-third of the American population and over half of doctors and hospitals. Anybody reading this would be tempted.

UDX is a biotechnology company founded in Spain in 2012, headquartered in Madrid, with locations in Ljubljana and Cambridge. Its Signal-C® identifies colorectal cancer tumor cells’ methylated DNA fragments in the blood for early screening. In May 2023, UDX presented study results at the Digestive Disease Week conference, showing Signal-C® achieved 93% sensitivity in colorectal cancer detection and 54% sensitivity for early adenomas under 92% specificity conditions.
Looks promising. Initially, UDX had little involvement in the U.S. market, but the irresistible allure of the American market led them to agreements similar to Geneoscopy and Labcorp. If Signal-C® passes FDA approval, Quest gains exclusive rights for its LDT tests in the U.S. To make the horse run, you need to let it eat grass. Correspondingly, our boss Quest will pay $70 million to UDX. After all, clinical work burns money, and offering some benefits is essential to ensure UDX’s determination to enter the U.S. market. With the money in hand, UDX is rejuvenated and decides to partner with Quest immediately.
To overcome the FDA hurdle, UDX plans to recruit over 15,000 patients at over 100 clinical sites across the U.S. The ambition and sincerity are evident.
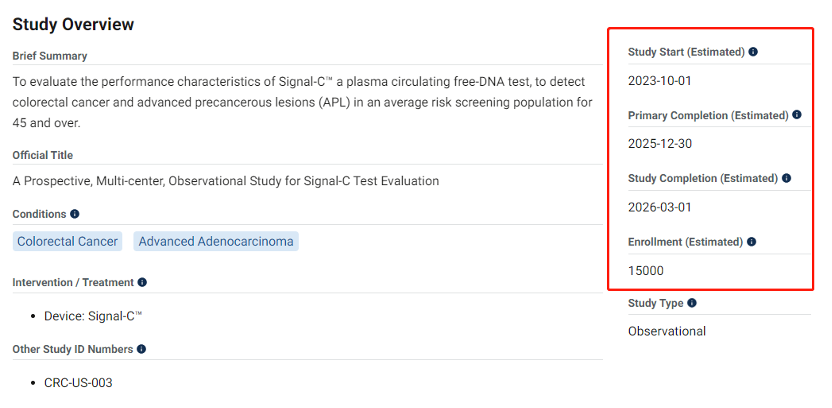
Speaking of Cologuard’s certification, how big was their clinical trial? In November 2012, Cologuard’s initial clinical data came from the DeeP-C study, recruiting over 12,700 subjects from 90 clinical sites in the U.S. and Canada. Well, UDX definitely didn’t match Cologuard on this front. Of course, Exact Sciences is not to be underestimated. Apart from the not-so-successful Epi Genomics acquisition, no one has truly threatened its position. Perhaps a fitting phrase to describe it these days is: “All roads lead to me.” Through storms and waves, it stands tall. UDX, born from blood tests, might not be an immediate threat to Exact Sciences, but with Quest’s support, Exact Sciences can’t ignore it. Everyone knows LDT is just a transition to certification. UDX will eventually grow to a point worthy of attention. In the meantime, with UDX standing before Exact Sciences, how will Exact Sciences, facing challenges that seem to never end, respond? Amidst a multitude of challengers, who can truly shake the dominant position of Exact Sciences?





